Quantum Chemistry
Using Gaussian® to learn about fate of additives
Electrolyte additives, chemicals added to electrolyte in very small amounts, can dramatically improve the performance and lifetime of cells. Many of these additives are observed to reduce at the negative electrode, indicating that the preferential reduction of additives leads to a passivating solid-electrolyte interphase (SEI), which hinders further parasitic reactions of the electrode with the negative electrolyte.
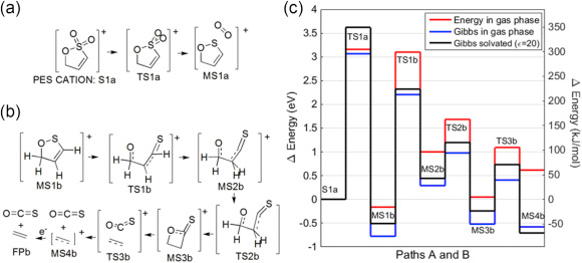
Computational chemistry can reveal the chemical reactions and the by-products that electrolyte additives produce inside a cell. This insight can lead to the intelligent design of new and improved electrolyte additives. The Figure below shows one path for the production of OCS gas from the electrolyte additive propene sultone (PES).
References:
- J. Self, D. S. Hall, L. Madec, and J. R. Dahn, J. Power Sources, 298, 369–378 (2015).
- D. S. Hall, J. Self, and J. R. Dahn, J. Phys. Chem. C, 119, 22322–22330 (2015).
- D. S. Hall, M. Nie, L. D. Ellis, S. L. Glazier, S. Hyatt, R. Petibon, A. Xiao, W. M. Lamanna, K. Smith, I. G. Hill, and J. R. Dahn, J. Electrochem. Soc., 163, A773–A780 (2016).
