Symmetric cells to study where additives act
Measuring the Impact of Electrolyte Additives using Symmetric cells
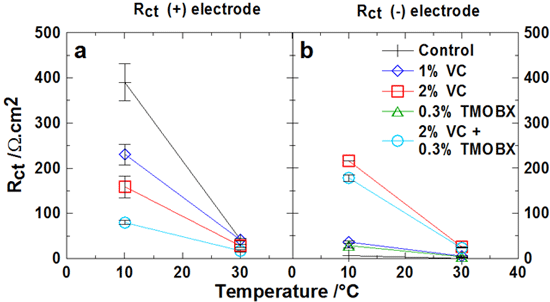
Electrolyte additives can greatly improve the cycle life and calendar life of Li-ion cells. In addition, additives can also reduce the impedance of Li-ion cells which improves their power capability. Additives modify the interface layers between the electrode materials and the electrolyte. Electrochemical impedance spectroscopy can be used to probe the resistance of these interface layers to the flow of Li ions. However, in a Li-ion cell it is hard to be sure how to separate the impedance into that from the positive interface and that from the negative interface. To do that, symmetric cells containing two positive electrodes or two negative electrodes are used. The Figure above shows the impact of various electrolyte additives on the positive electrode (panel a) and negative electrode (panel b) charge transfer resistances.